Colorful Crystals
Minerals can come in a wide variety of colors. Minerals such as gold always have the same color. Some mineral species, such as quartz and calcite, can come in a variety of colors.
Rainbow Minerals
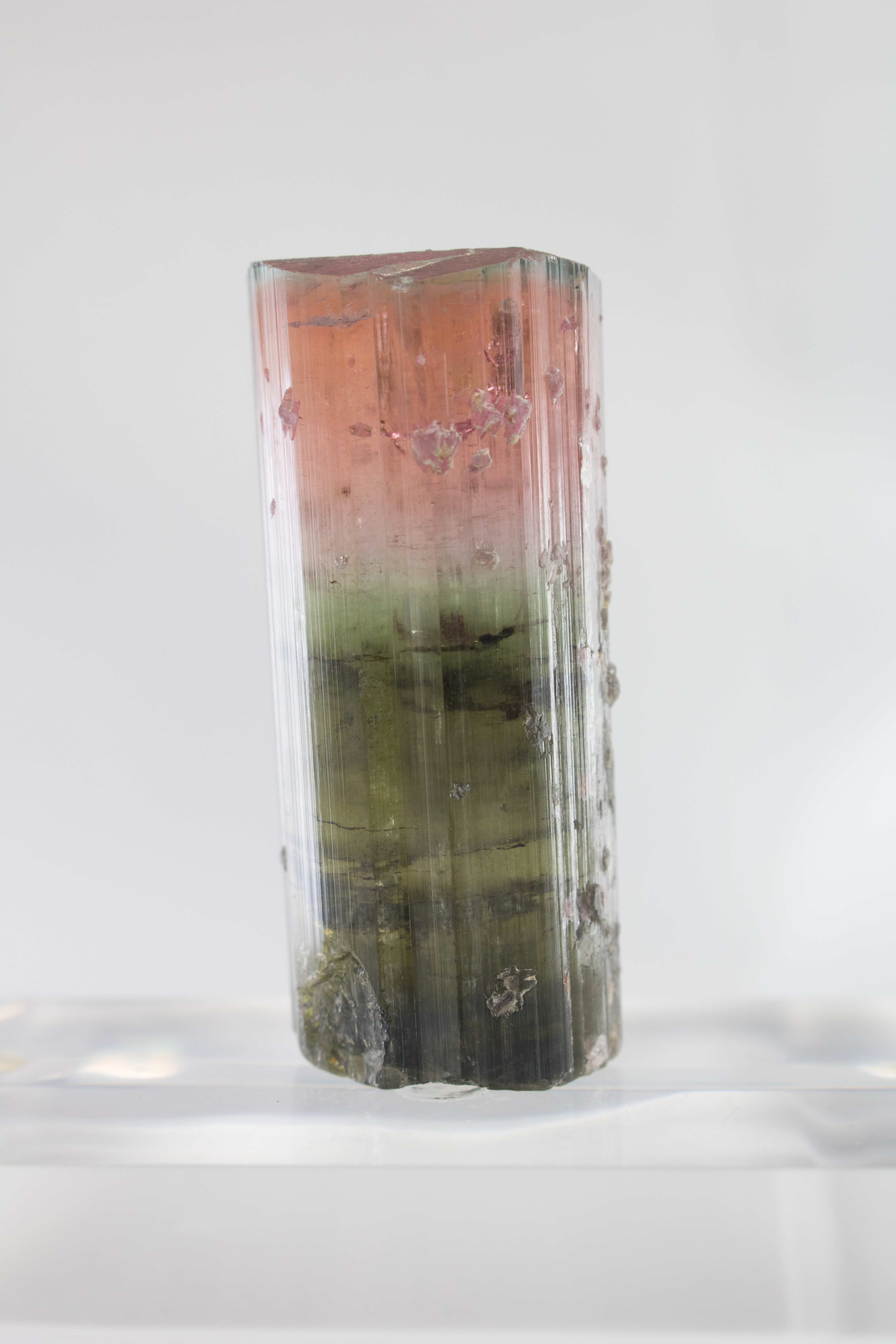
Certain minerals form in various colors. In fluorite and tourmaline, a single crystal can form two or more colors.
Minerals with single color varieties:

Calcite (red, golden, gray, white, green, black)

Quartz (clear, smoky, orange, purple) Some color
variations of quartz also come with a variety of names:
yellow/orange quartz is Citrine and purple quartz is Amethyst.
Some minerals that exhibit a variety of colors sometimes show them within the same crystal:
- Tourmaline (var. Elbaite)

- Fluorite

Fluorite (single green, shades of blue, purple,
and blue, single purple). There are a variety of
methods by which minerals get their color.
Element Substitution
Color variability in garnets is due to element substitutions. In andradite garnet, iron causes the mineral to appear dark red. In grossular garnet, aluminum is present instead of iron, resulting in a yellow-green color.


Trace Element
The color variation in some minerals is due to trace elements. Trace elements are elements that are not part of the chemical composition but are present in small amounts and able to influence the color. Iron can cause some minerals to be other colors. In amethyst, there are trace amounts of iron (Fe) that cause quartz to appear purple. Traces of iron (Fe2+) in the mineral beryl produce the light-blue color of the beryl variety aquamarine.


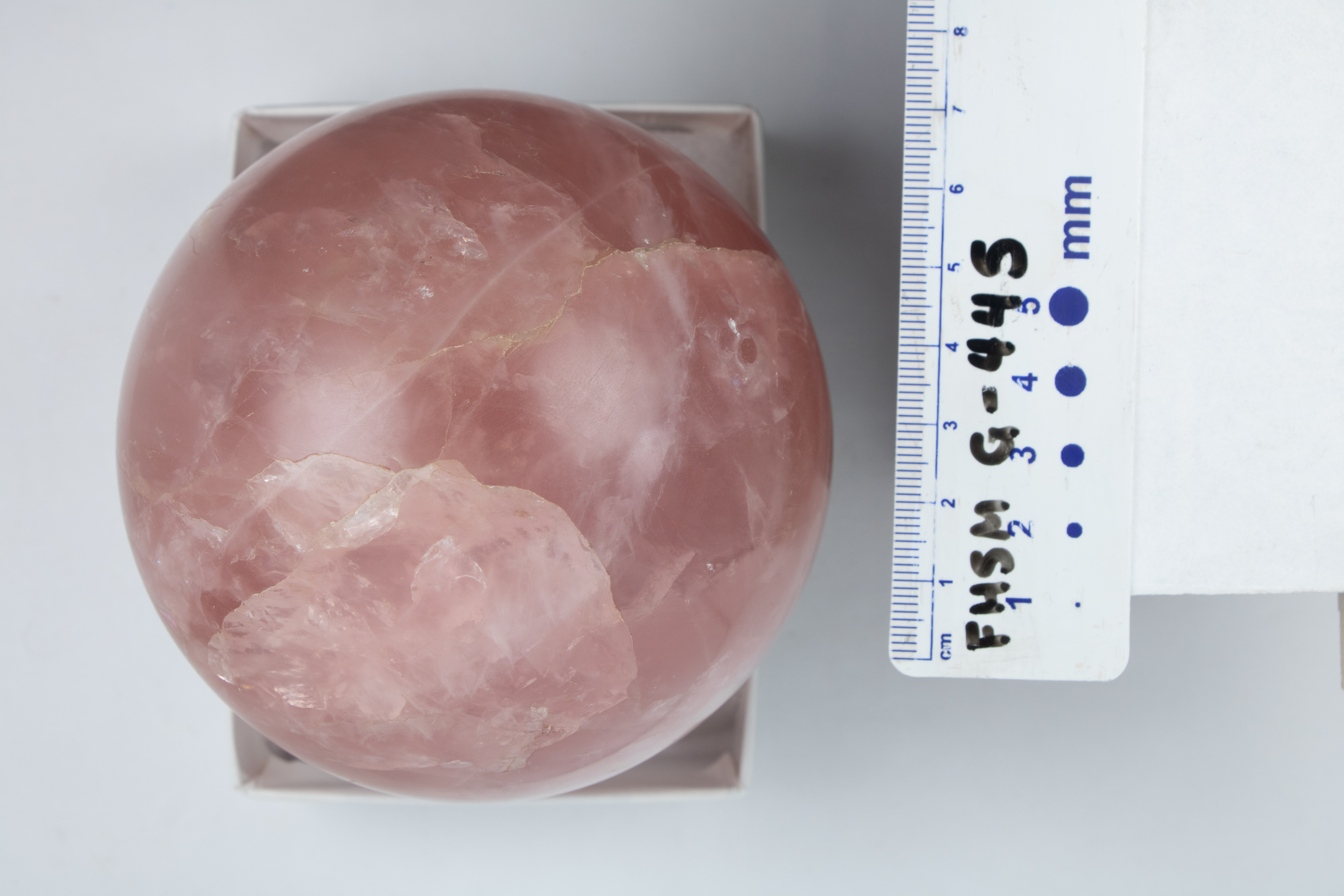
Rose Quartz – The pink color comes from trace amounts of titanium, iron, or manganese, in the mineral. Rose quartz is commonly found in the quartz cores of pegmatites and is believed to form at high temperatures, but it has also been found in hydrothermal veins. They are found as a late formation in pegmatite pockets, often overgrowing smoky quartz crystals in groups of parallel-grown crystals.
Smoky Quartz – The dark color comes from irradiation that changes how the trace elements relate to one another at the electron level within quartz.

Effects of Oxygen
Oxygen affects minerals through a weathering process called oxidation. Oxidation is a chemical reaction that takes place when a mineral is exposed to oxygen. When iron and oxygen react in the presence of water or moisture in the air, rust forms. Rust will continue to form until one of the main ingredients (in this case, the iron) runs out. Therefore, a piece of iron left in a weathering environment (e.g. outdoors) will continue to rust until the metal is completely consumed.

Rusted Meteorite – Rust is an iron oxide produced
through the oxidation of iron. (Chemical composition: FeO)
Minerals, like silver, can be affected by oxygen and acids that result in tarnish. Tarnish is a thin layer of corrosion that changes the top few layers of the metal, preserving the lower layers from further tarnish. This layer of tarnish is called patina. Because tarnish affects only the top layers, it can be removed, revealing the original metal below.


